Cell culture represents a key tool in modern scientific research. Over the past decades, scientists have developed advanced cell culture techniques to isolate individual cells from tissues and obtain homogeneous cell populations that can be maintained, manipulated, and multiplied in vitro.
The 4 types of cell culture: definition, differences, and application examples
Understanding the different types of cell culture is essential, as each presents unique characteristics and is suited to specific experimental or production goals.
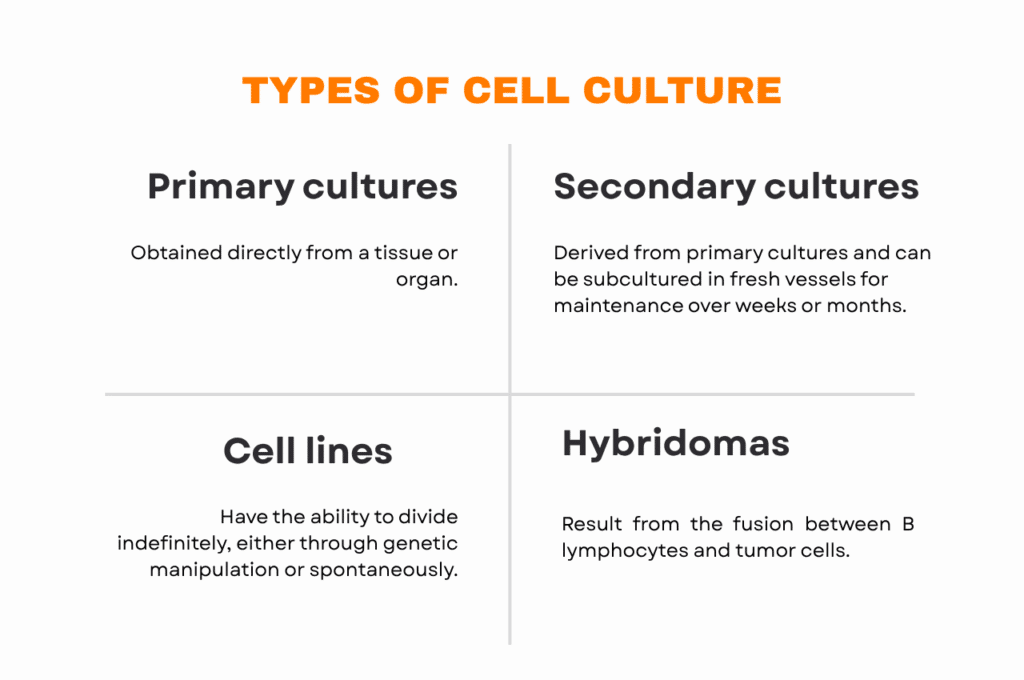
Primary cultures
These are obtained directly from a tissue or organ. They retain the original characteristics of the source tissue, although their proliferation capacity is limited. Primary cultures are often used in early research stages and can give rise to secondary cultures.
Secondary cultures
Derived from primary cultures, these can be subcultured in fresh vessels for maintenance over weeks or months. Secondary cultures not only allow cells to be maintained for extended periods but also enable the study of specialized functions such as intestinal absorption or compound metabolism.
For example, this type of cell culture can be applied in in vitro bioavailability assays using human intestinal cell lines like Caco-2, to evaluate how functional ingredients, nutraceuticals, pharmacological, or bioactive compounds are absorbed after passing through a dynamic digester simulating the human gastrointestinal process.
Cell lines – continuous cultures
Some cells can acquire the ability to divide indefinitely, either through genetic manipulation (such as the introduction of telomerase or oncogenes) or spontaneously, as in many rodent cells. These cell lines are widely used due to their stability, homogeneity, and ease of storage in liquid nitrogen. They can also be cloned to generate genetically identical populations, which allows researchers to study specific mutations.
Hybridomas
Resulting from the fusion between B lymphocytes and tumor cells, hybridomas are cell lines capable of producing monoclonal antibodies indefinitely. This technology makes it possible to obtain highly specific antibodies for the treatment and detection of multiple diseases.
How are cells obtained in cell culture?
Cell culture refers to the set of techniques that allow living cells to be maintained outside their organism while preserving their physiological properties. These cells can grow either in suspension or as a monolayer and can be obtained from various biological sources.
To establish cultures from tissues, the first step is to isolate individual cells by separating them from the extracellular matrix that keeps them together. This is achieved using proteolytic enzymes and chelating agents such as EDTA, which interfere with cell adhesion. The result is a mixed cell suspension that can then be purified according to the desired cell type.
Common methods for separating cell types include:
- Centrifugation, based on cell size.
- Differential adherence to glass or plastic surfaces.
- Specific antibodies bound to solid matrices (such as latex or collagen beads).
- Labeling with fluorescent antibodies and separation by cell sorting.
- Laser microdissection, useful for isolating specific cell groups from complex tissues such as tumors.
Bioavailability assessment using cell culture
The use of human intestinal cell culture models such as Caco-2 has become an advanced tool for in vitro bioavailability testing. When combined with dynamic gastrointestinal digestion systems, these models can realistically simulate the human digestive process, providing detailed information on how bioactive compounds are released, absorbed, and metabolized.
This methodology is especially valuable for assessing the efficacy of functional ingredients, nutraceuticals, and pharmacological compounds, while significantly reducing both costs and development times in early research phases.