In the development and manufacturing of medicines—especially in regulated markets such as the European Union—it is increasingly common to outsource part of the process to specialized companies. This is where two key players come into focus: CMOs (Contract Manufacturing Organizations) and CDMOs (Contract Development and Manufacturing Organizations). Although often used interchangeably, their scope and functions differ. Understanding that difference is essential to selecting the right partner based on the product’s development stage and the pharmaceutical company’s goals.
What Does CDMO Mean in Pharma?
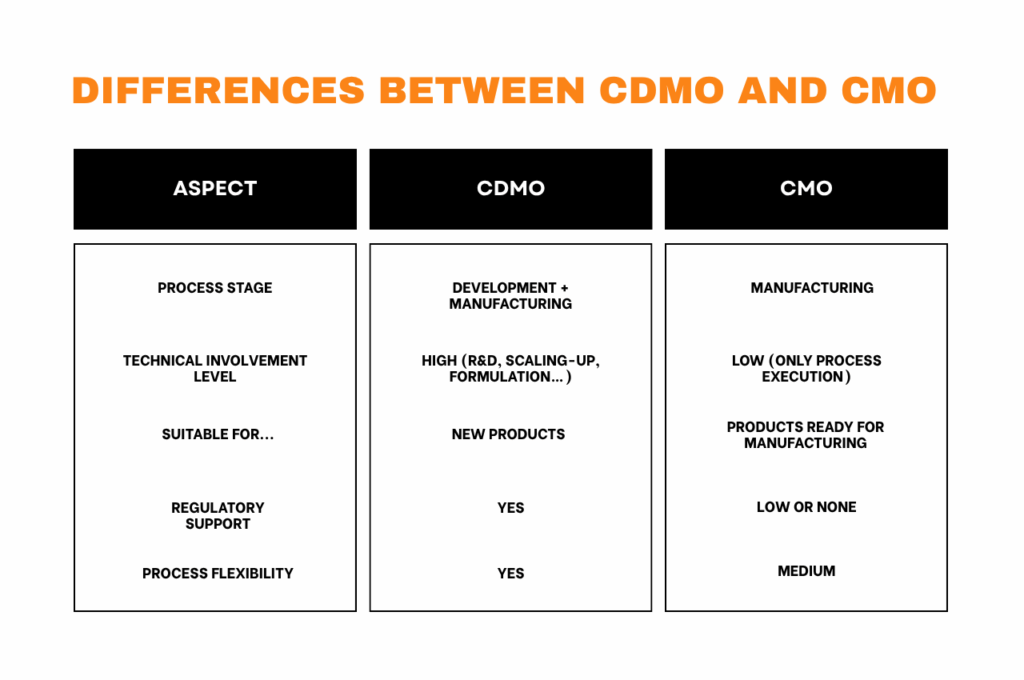
A CDMO is a company that offers integrated services in both the development and manufacturing of pharmaceutical products, covering everything from chemical formulation and industrial scale-up to commercial production under GMP standards.
Key functions:
- Pre-formulation development, analytical methods, stability studies, and final API formulation.
- Process scale-up (from grams to tons) and manufacturing of clinical and commercial batches.
- Regulatory support and technology transfer.
- Packaging, logistics, and supply chain management.
CDMO pharma services help pharmaceutical companies accelerate time-to-market, reduce costs and risks, and focus on core R&D while outsourcing critical technical and operational phases.
CMO Meaning in the Pharmaceutical Industry
A CMO focuses on contract manufacturing, providing production services for already developed pharmaceutical products, without participating in formulation or technical development.
Key functions:
- Production of APIs, solid oral forms, liquids, injectables, and sterile products.
- Minimal preformulation and execution of validated processes, including packaging and labeling.
- Quality assurance, GMP compliance, and full traceability.
CMOs are ideal for companies that have finalized product development but need industrial capacity, production flexibility, or access to GMP-certified facilities.
Key Differences Between CDMO and CMO in Pharma
1. Scope of Services
CDMO: Offers an end-to-end service that covers everything from preclinical development (formulation, API optimization, analytical methods) to full-scale GMP manufacturing, including scale-up, clinical trial support, and regulatory compliance.
CMO: Focuses exclusively on manufacturing, working with pre-developed and validated formulations without participating in R&D stages.
2. Drug Lifecycle Stage
CDMO: Is involved from the earliest stages of the project, helping to design and optimize molecules or formulations before scaling up.
CMO: Steps in at later stages, when clinical or commercial batch production is needed based on an already defined formulation.
3. Level of Technical Involvement
CDMO: Provides comprehensive technical expertise, from research and development to regulatory strategy, clinical trial management, and technology transfer.
CMO: Is limited to executing validated processes, ensuring quality and traceability but without contributing to product development.
4. Strategic Benefits
CDMO: Accelerates time-to-market, reduces financial risk by avoiding internal infrastructure investment, and lowers costs through an “all-in-one” model.
CMO: Offers production flexibility, access to installed capacity without capital investment, and is ideal for pure manufacturing phases.
5. Technology and Regulatory Transfer
CDMO: Manages technology transfer, process validation, and regulatory support for clinical trials and market authorization.
CMO: Typically works with pre-approved processes, while also ensuring GMP compliance and meeting client contractual obligations.
Best Practices to Ensure Product Quality
Choosing the right partner and applying best practices from the start is key to ensuring pharmaceutical quality, regulatory compliance, and cost-efficiency. Here are a few recommendations:
- Technical capability validation: Ensure the provider has experience with the product type (oral, injectable, biologic) and required scale.
- Regulatory compliance: Demand GMP certifications, full traceability, and robust quality control systems.
- Process transparency: Maintain clear communication, confidentiality agreements, and IP protection frameworks.
- Clean and efficient technologies: Leverage technologies like supercritical CO₂ extraction, especially during early development phases, to obtain APIs that are purer, more sustainable, and compliant with EU regulations.
When Can AINIA Act as a CMO or CDMO?
At AINIA, we bring extensive experience in the development and production of active pharmaceutical ingredients (APIs), allowing us to act as a CDMO pharma or CMO, depending on each client’s specific needs.
- As a CDMO, we offer end-to-end services—from API design and optimization using supercritical CO₂ extraction to process scale-up, characterization, validation, and regulatory support. This is especially beneficial during early-stage development or when adapting to strict EU regulatory frameworks.
- As a CMO, we support companies with finalized formulations that require a partner for safe, efficient, and GMP-compliant manufacturing—whether for pilot-scale batches or industrial production.
Thanks to platforms like ALTEX, and our ability to adapt to different production scales and regulatory demands, we provide flexible and sustainable solutions tailored to innovation-driven pharmaceutical companies.