Understanding, evaluating, and demonstrating the mechanism of action of functional ingredients is essential for companies in the sector. Society increasingly turns to these compounds to complement nutrition, but their inclusion in a formulation is not enough. It is crucial to justify their use with scientific criteria: they must be selected rigorously and supported by solid research, development, and validation of the functional effect attributed to them.
How does an active ingredient behave within the digestive system?
When we ingest food or supplements, their active ingredients must overcome a complex journey through the digestive process. Throughout this journey, they can undergo chemical transformations, interact with digestive enzymes, be affected by pH variations, or come into contact with the intestinal microbiota.
It is therefore essential to know:
- Whether the ingredient resists digestive conditions or degrades before exerting its effect.
- In what form it reaches the intestine and whether it is bioavailable — that is, absorbable.
- What impact it has on the intestinal microbiota and whether it generates bioactive metabolites.
This knowledge makes it possible to design more effective products and objectively substantiate their claimed benefits.
The digestive process step-by-step
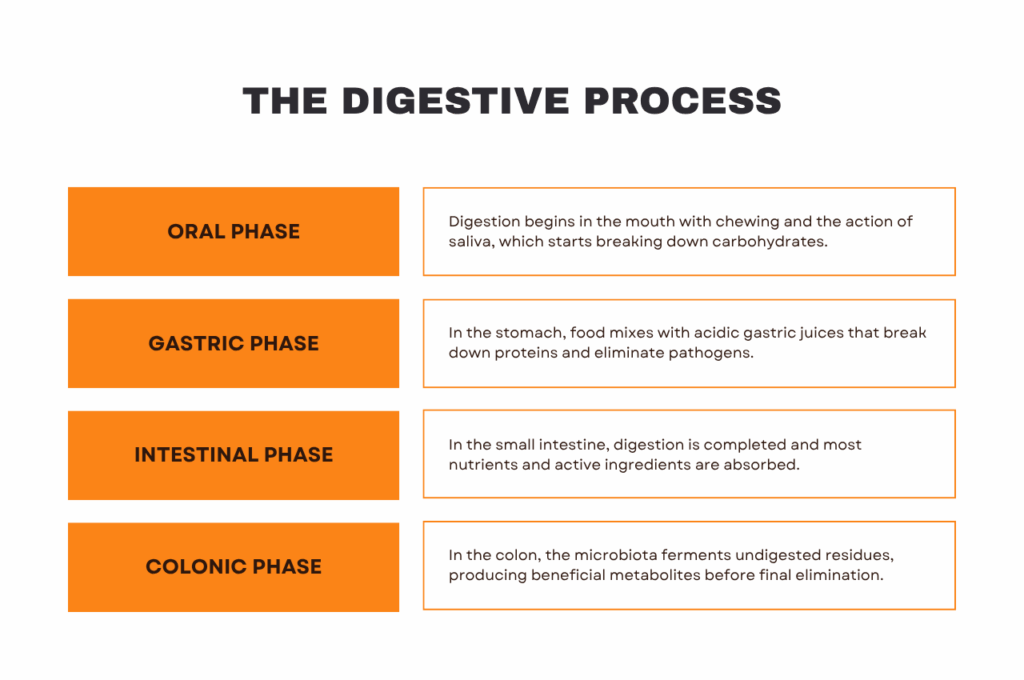
The human digestive system is divided into several phases, each with very different conditions that can influence the behavior of an active ingredient.
 Oral phase
Oral phase
Digestion begins in the mouth, known as the oral phase. Here, the teeth grind food into smaller particles, facilitating its subsequent processing. At the same time, the salivary glands play a key role by secreting enzymes such as salivary amylase, which begins to break down complex carbohydrates.
Once properly chewed and mixed with saliva, the food becomes the bolus, which travels down the esophagus to the stomach, leading to the gastric phase.
Gastric phase
When the bolus reaches the stomach, the gastric phase begins, characterized by a highly acidic environment. Here, food is mixed with gastric juices, a combination of hydrochloric acid and digestive enzymes, primarily pepsin, which breaks down proteins into smaller fragments.
This stage performs key functions:
- Denatures proteins, facilitating their digestion.
- Activates specific enzymes.
- Eliminates pathogenic microorganisms due to the low pH.
The duration of this phase varies between 2 and 4 hours, depending on the type of food ingested (for example, proteins take longer than simple carbohydrates). The result is a semi-liquid mixture called chyme, which is gradually released into the small intestine to continue the digestive process.
Intestinal phase
In the intestinal phase, the chyme passes into the small intestine, where most digestion and nutrient absorption occur. It is also the most relevant stage for studying nutrient absorption and determining the potential effects of an active ingredient or functional compound.
This stage of the digestive process is driven by the combined action of pancreatic enzymes and bile salts, which break down fats, proteins, and carbohydrates into simpler molecules ready to be absorbed by the body.
During this phase:
- The most intense enzymatic digestion takes place.
- Vitamins, minerals, amino acids, fatty acids, and simple sugars are absorbed.
- The intestine regulates the bioavailability of active or functional ingredients.
This phase may last between 4 and 6 hours, depending on the type of food and the physiological state of the person.
Colonic phase
After most nutrients have been absorbed in the small intestine, undigested material passes into the large intestine, beginning the colonic phase. From a scientific perspective, this stage is crucial to assess whether a functional ingredient has a prebiotic effect, modulates the microbiota, or generates beneficial metabolites.
These effects can be studied in a controlled way using gastrointestinal simulation models, which accurately reproduce the entire digestive process and provide detailed information on how ingredients impact the microbiota and its metabolism.
This stage includes two key processes: microbial fermentation and elimination.
In the colon:
- Water and some residual minerals are absorbed, helping to compact intestinal contents.
- The intestinal microbiota acts on undigested compounds, generating metabolites such as short-chain fatty acids (SCFAs), which play a crucial role in metabolic, immune, and intestinal health.
Understanding this phase in depth enables progress toward solutions for more complex problems, such as chronic intestinal inflammation or colorectal cancer. The contents can remain in the colon for 12 to 24 hours before being expelled through the rectum as feces.
Why Use In Vitro Models to validate active ingredients?
To obtain scientific evidence about the real effect of an active ingredient in the body, in vitro digestion models have become an essential tool. These tests allow researchers to reproduce, in a controlled way, the physiological conditions that occur throughout the digestive process — from the oral phase to colonic fermentation.
Dynamic in vitro models accurately reproduce key variables such as pH, transit time, the presence of digestive enzymes, and salt concentration at each stage of the digestive system.
In addition to enabling the study of an ingredient’s behavior along the gastrointestinal tract, they provide an effective means to analyze its potential functional or beneficial effects after digestion and absorption. This methodology is also successfully applied in the field of animal digestive health, where in vitro digesters allow the evaluation of functional ingredients without resorting to animal testing.
These assays typically cover:
- Oral phase, where the initial release of the ingredient is evaluated.
- Gastric phase, to study its stability under acidic conditions.
- Intestinal phase, critical for determining its bioavailability.
- Colonic phase, which allows analysis of microbial fermentation and production of active metabolites.
After digestion, intestinal absorption can also be simulated using cell culture models such as the Caco-2 cell line, representative of the human intestinal epithelium. This technique helps estimate which fraction of the ingested compound could actually reach the bloodstream (the bioavailable fraction), which is crucial for ingredients with systemic effects.
Thanks to their versatility, reproducibility, and lower cost compared with in vivo studies, these models are increasingly used in both research and the development of new nutraceutical, functional, and veterinary products.


 Oral phase
Oral phase


